Monday
13
Staff Development Day
No School!!!
14
Staff Development Day
No School!!!
Target - Students will learn what the "SPIRIT" of Great Oak means.
SPIRIT Day!!!
S - Scholarship
P - Passion
I - Integrity
R - Reflection
I - Involvement
T - Teamwork
Minimum Day
Target - Students will become familiar with classroom content, standards, procedures and safety.
Introductions--Go over basic expectations
Lunch on me--Describing observations
HW Show parents the syllabus and get it signed --signature sheet
Unique: GOHSChem - https://quest.cns.utexas.edu/student/courses/enroll_by_link?courseunique=148769
Sign Up for Canvas
Target - Student will understand safe lab practices
-Go to lab
Safety and First Aid
Accident at Jefferson High Video
Watch this fun video on You tube. Maybe you can do it in class for us?
HW. Sign Flinn Safety sheet (Same one handed out during class.)
Unique: GOHSChem - https://quest.cns.utexas.edu/student/courses/enroll_by_link?courseunique=148769Sign Up for Canvas
20
Late Start
Target- Students will review the scientific method
Mind Meld --Scientific Method
FIRST ONLINE HW DUE Thursday Night!
---Used in class.
Chapter 1 Powerpoint --The Importance of Learning Chemistry--Complete
Chemistry 101: The Scientific Method
 Note
Taking Guide
Note
Taking Guide
21
Target--Students should be able to determine which variable in an experiment is the Independant / Manipulated and which is the Dependant as well as write a good question for an experiment.
22
Target--Students should be able to distinguish between elements, compounds and mixtures. State which elements come as Diatomic (BrINClHOF)
Elements, Compounds and Mixtures --Video
Kind of fun Lego on classification of matter
Chart.pdfchapter 2 classification of matter
Try some of these activities Click Here to help you learn the elements.
chapter 2 ppt-complete chapter
Chemistry Ch. 1 & 2 Take Home Test Due Wednesday night!!!--Chapter 1 and 2 Test Next Friday
23
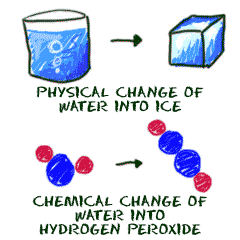
Target- Students should be to define a physical and/or chemical change. Students should be identify if a change is physical or chemical in nature given examples
Physical and Chemical Changes -Exploding whale video
Physical vs Chemical Change animation.swf
Physical and Chemical Changes ppt
States of Matter and their properties video
Chemical and Physical Properties and Changes Video
Chemistry Ch. 1 & 2 Take Test--Due Wednesday night!!!
HW.. Elements, Compounds and Mixtures / Properties / Changes Quest Quiz Due tonight by midnight
24
Please identify if the part is demonstrating a Physical of Chemical Change and explain why.
Chemistry Ch. 1 & 2 Take Home Test--Due Wednesday night!!!
Please identify if the part is demonstrating a Physical of Chemical Change and explain why.
Work on lab
Target--Students should be able to determine which variable in an experiment is the Independant / Manipulated and which is the Dependant as well as write a good question for an experiment. Students should be able to organize data and know which axis is the independant and dependant variables,
Target - Students should be know the targets for Chapter 1 and 2
Target: Students should be know the targets for Chapter 1 and 2
Chemistry Ch. 1 & 2 Take Home Quiz--Due tonight!!!
Go over key terms!
List of the Elements to Memorize by next Thursday.
Element Song Live - by Tom Lehreret.pdf
Chapter 1 and 2 Quiz
List of the Elements to Memorize by next Thursday.